Concerns over the potential risk of eye infection have led to the recall of eye ointment products sold at Walmart and CVS stores across the United States.
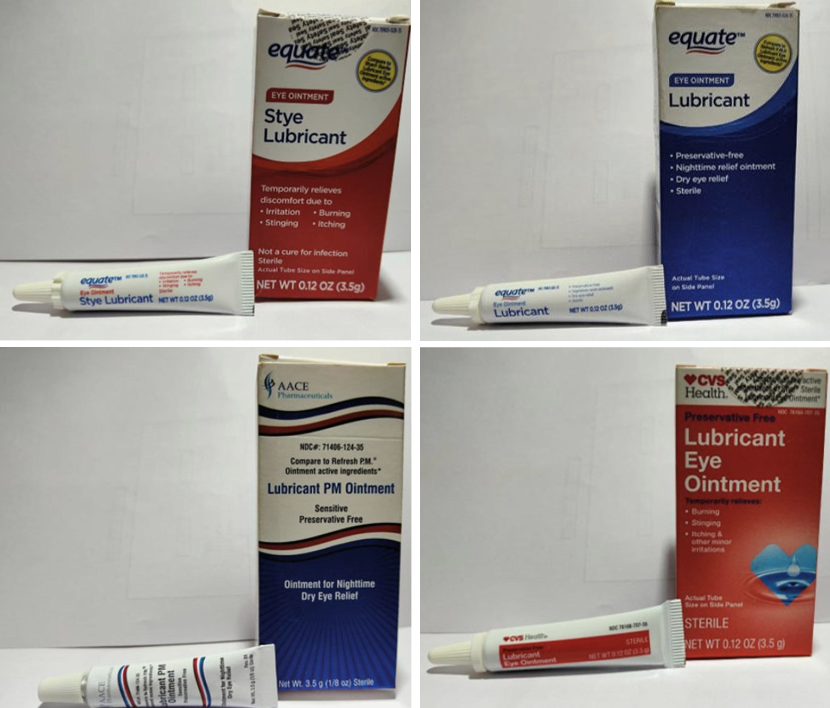
In an announcement posted on the U.S. Food and Drug Administration's website, India-based Brassica Pharma Pvt. Ltd. said it is recalling four types of eye lubricant "due to lack of sterility assurance" at the facility where they were manufactured. The ointments are sold under the brand names Equate, CVS Health and AACE Pharmaceuticals.
The company said people who use the lubricants could be at risk for infections or related harm, but no injuries have been reported as of Feb. 16. Consumers have been advised to immediately stop using the products and return them to where they were purchased.
A full list of recalled ointments is listed below:
Equate
Equate Lubricant Eye Ointment (Mineral Oil 42.5%, White Petrolatum 57.3%, Lanolin Alcohols)
- 3.5 gram tube, packaged in cardboard box with UPC Code 681131395298
- National Drug Code: 79903-026-35
- Lot Numbers: A2E01, A2L05, A3B01, A3C01, A3H05
- Expiration Dates: April 2024, Nov. 2024, Jan. 2025, Feb. 2025, July 2025
Equate Stye Lubricant Eye Ointment (Mineral Oil 31.9%, White Petrolatum 57.7%, Microcrystalline Wax, Stearic Acid, Wheat Germ Oil)
- 3.5 gram tube, packaged in cardboard box with UPC Code 681131395304
- National Drug Code: 79903-028-35
- Lot Numbers: A2D08, A2F02, A2I03, A2L03, A2L04, A3C03, A3C05, A3H01, A3H03
- Expiration Dates: March 2024, May 2024, Aug. 2024, Nov. 2024, Feb. 2025, July 2025
CVS Health
CVS Health Lubricant Eye Ointment (Mineral Oil 31.9%, White Petrolatum 57.7%, Microcrystalline Wax, Stearic Acid Wheat Germ Oil
- 3.5 gram tube, packaged in cardboard box with UPC Code 050428634141
- National Drug Code: 76168-707-35
- Lot Numbers: A2F03, A2I02, A2L02, A3C04, A3H04
- Expiration Dates: May 2024, Aug. 2024, Nov. 2024, Feb. 2025, July 2025
AACE Pharmaceuticals
AACE Pharmaceuticals Lubricant PM Ointment
- 3.5 gram tube, packaged in cardboard box with UPC Code 371406124356
- National Drug Code: 71406-124-35
- Lot Numbers: A2G01, A2G02, A3F08, A3F09, A3J17, A3J18
- Expiration Dates: June 2024, May 2025, Sept. 2025
Consumers who experience adverse reactions after using these products can report it to the FDA here.
Trending stories at Scrippsnews.com


